Gras is an acronym for the phrase generally recognized as safe under sections 201 s and 409 of the federal food drug and cosmetic act the act any substance that is intentionally added to.
Fda mat list.
Government responsible for determining how materials may be caused in contact with food products.
Components of a food packaging material used in compliance with a regulation in 21 cfr 174 179 need no further fda review.
Most of the regulated indirect food additives can be found in cfsan s.
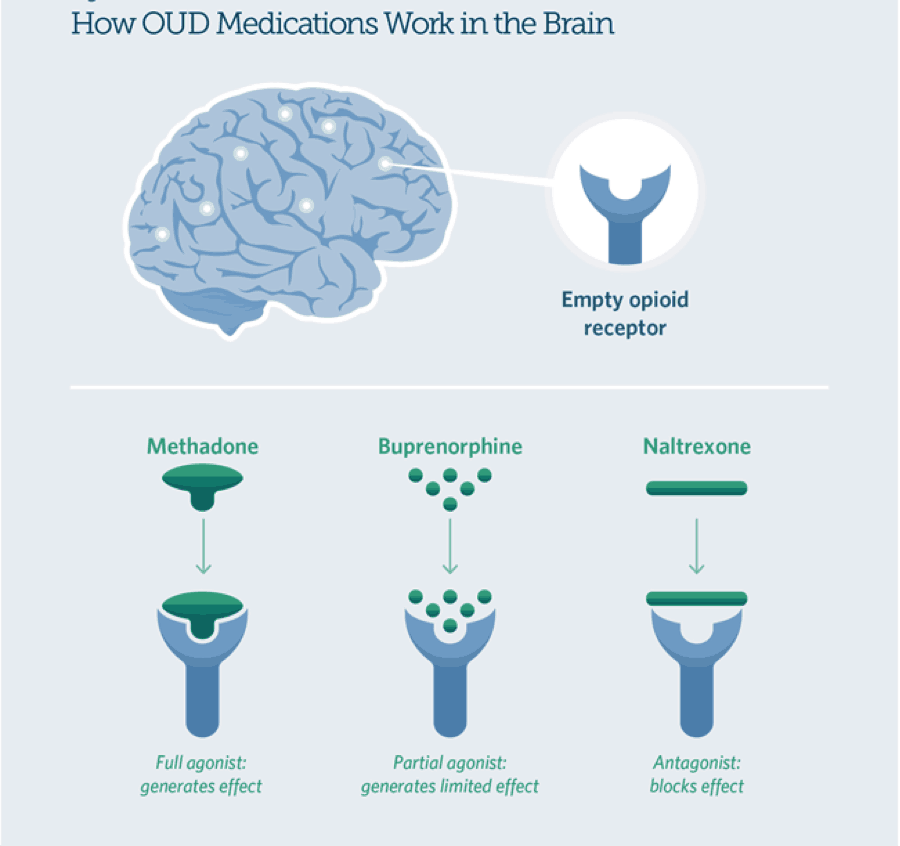
Medications used in mat are approved by the food and drug administration fda and mat programs are clinically driven and tailored to meet each patient s needs.
Piece of hardware or software technology or new it project to the fda.
On top of creating stringent guidelines for consumable items they also look closely at things like preparation equipment and packaging materials including plastic.
Master approved technology list mat list.
Contact fda follow fda on facebook follow fda on twitter view fda videos on youtube subscribe to fda rss feeds fda homepage contact number 1 888 info fda 1 888 463 6332.
The food and drug administration fda was created to set standards for the safe production and storage of food beverages and drugs.
Medication assisted treatment mat is the use of medications in combination with counseling and behavioral therapies to provide a whole patient approach to the treatment of substance use disorders.
A listing of potentially approved technologies for use throughout the fda.
Medications used for mat are evidence based treatment options and do not just substitute one drug for another.
The fda participates in publication of the the federal register which contains the code of federal regulations cfr a codification of the general rules established by the.